
News

News

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation
Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

MD, AI and Cybersecurity

Medical devices regulation

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

MD, AI and Cybersecurity

News

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

News
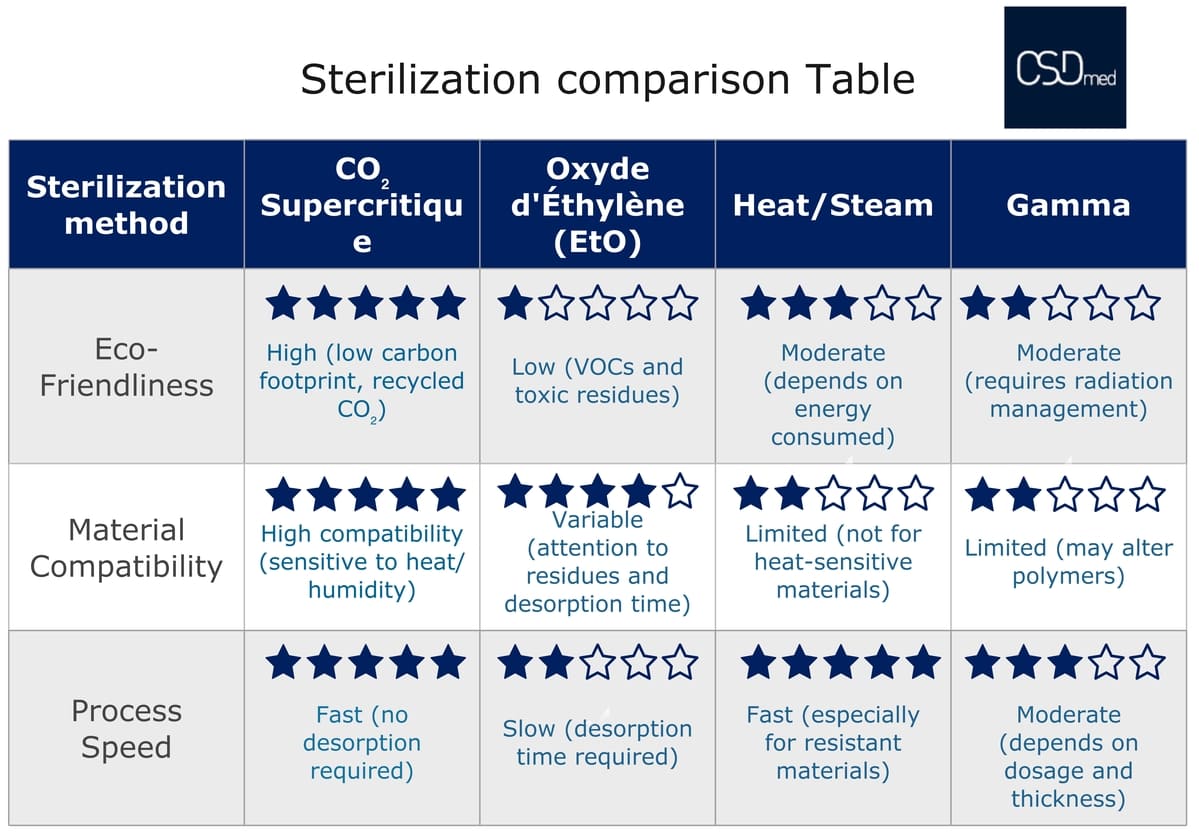
Medical devices regulation

Medical devices regulation

Medical devices regulation

MD, AI and Cybersecurity

MD, AI and Cybersecurity

MD, AI and Cybersecurity

News

News

Medical devices regulation

MD, AI and Cybersecurity

MD, AI and Cybersecurity

News

Medical devices regulation

Medical devices regulation

News

Medical devices regulation

News

Medical devices regulation

MD, AI and Cybersecurity

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation
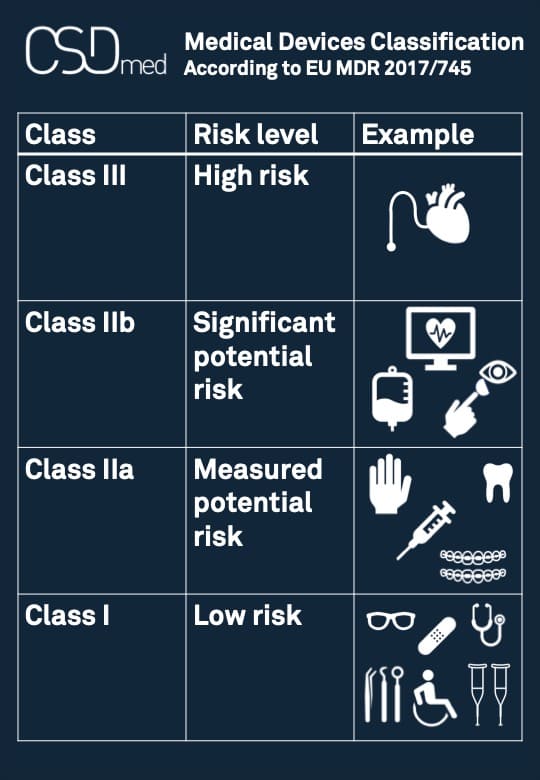
Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

News

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation